Won’t seek care due to fear of withdrawal
With existing beds, time current system needs to detox OUD pop.4
What if you had an opioid withdrawal treatment that offered:
FDA-Cleared Safe, Comfortable, Drug-Free
Sparrow Ascent Drives Organization Wide Opportunity To Enhance Care
Level 1, RCT NIH: NIDA Published
Non-Addictive, No Diversion Risk, No Known Side Effects
5 Min Activation, No Needed Withdrawal, Zero Needles
No X-Waiver Hosp. to Home Alone or Added
>50% ↓ in 60 Min 100% of Patients COWS, PCL-5, PHQ-9
Robust Training Dedicated Team 24/7 Care
Everything Needed for Therapy - All In One Kit
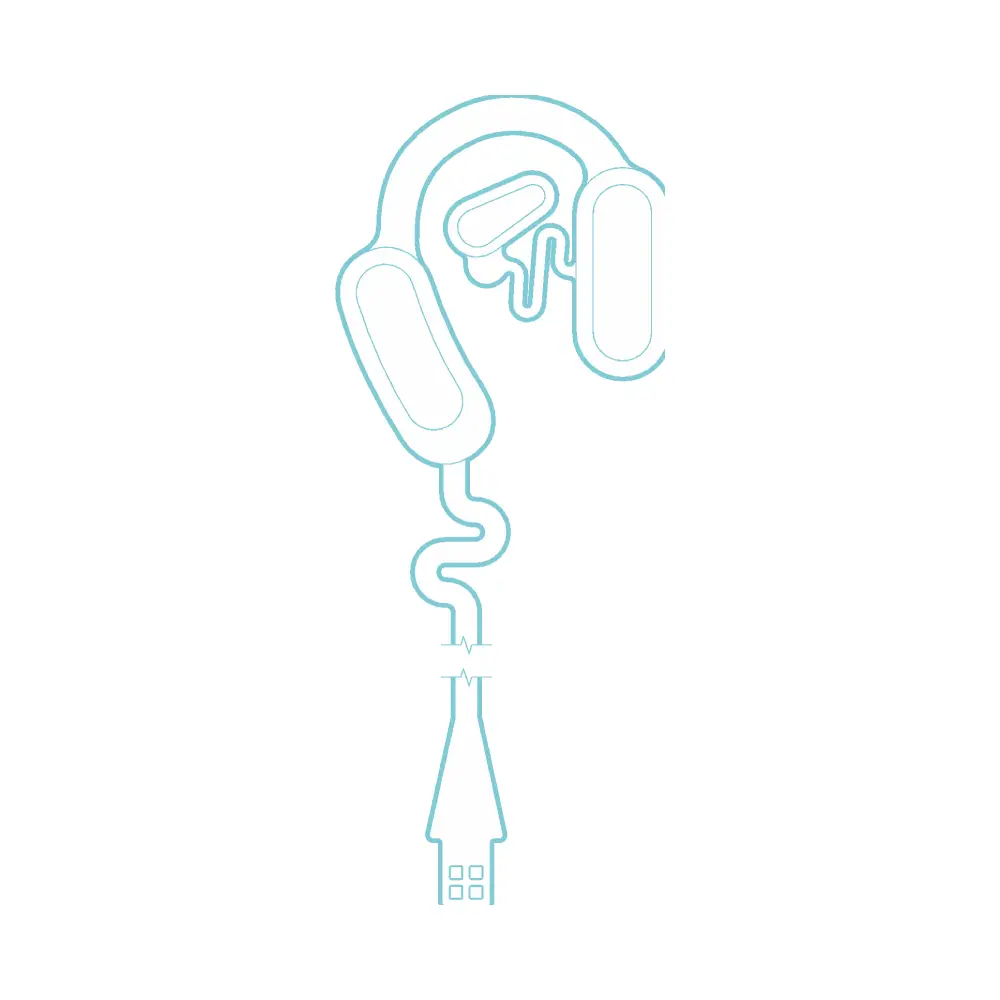
They’re easy to apply, just like a sticker, and deliver mild electrical stimulation to the nerves on and around the ear.
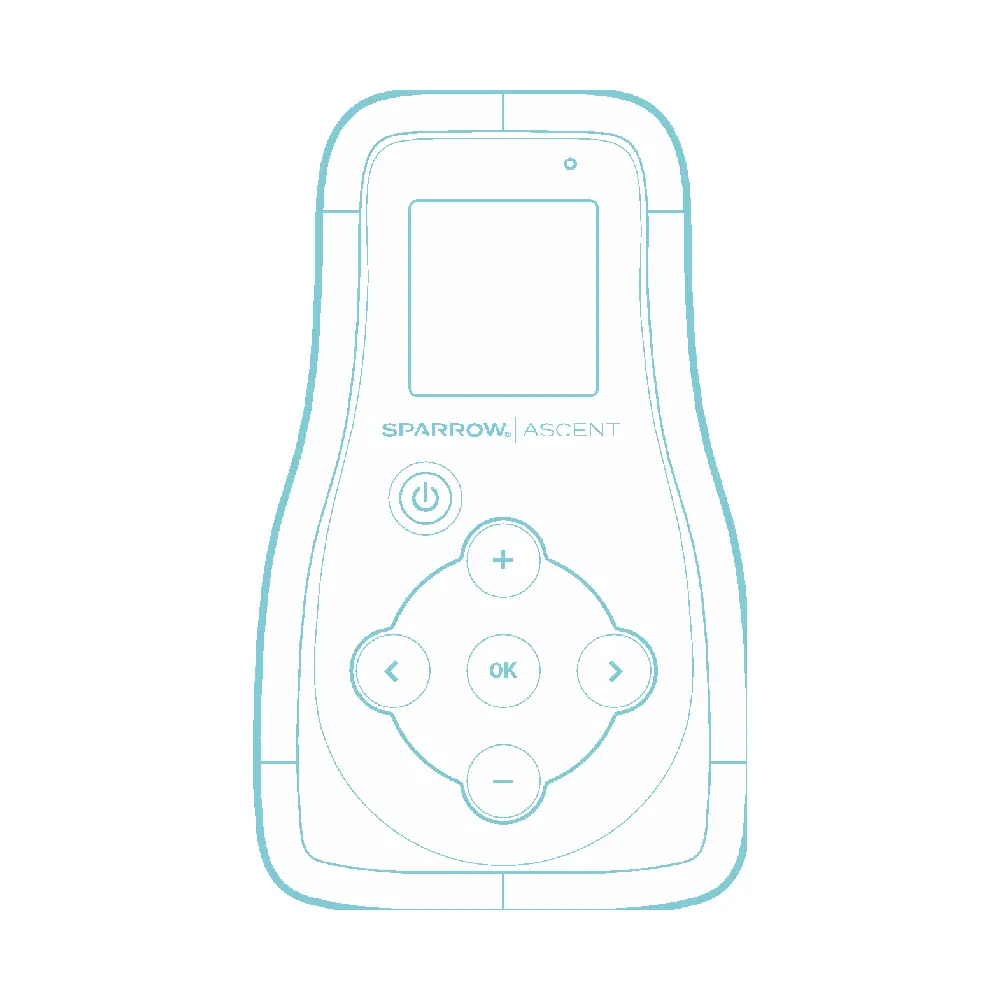
It powers the Earpiece and puts the patient in control of the duration and level of stimulation needed for relief.
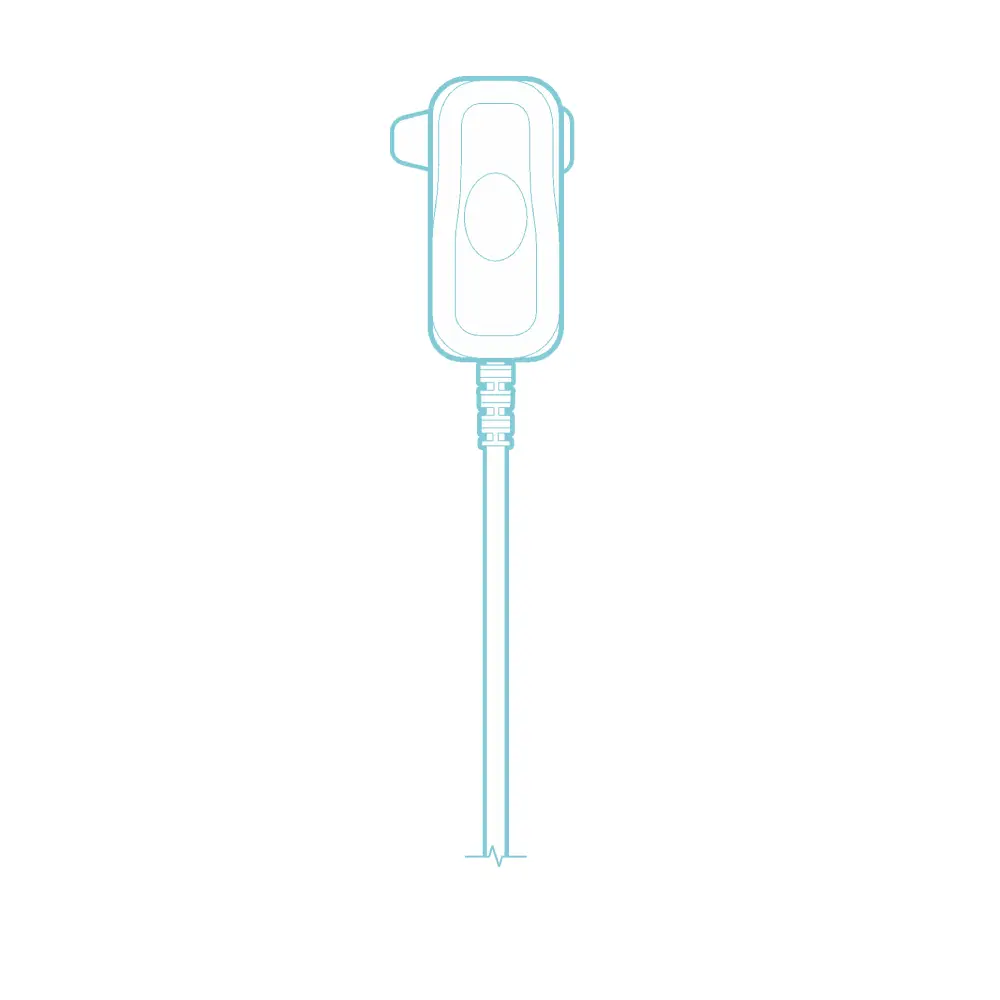
It connects and delivers electrical pulses from the Patient Controller to the Earpiece.
1 Meade, C.S., Lucas, S.E., McDonald, L.J., Fitzmaurice, G.M., Eldridge, J.A., Merrill, N., Weiss, R.D., (2010) A randomized trial of transcutaneous electric acupoint stimulation as adjunctive treatment for opioid detoxification. Journal of Substance Abuse Treatment, 38(1), 12-21., Han, J. S., & Wang, Q. (1992). Mobilization of specific neuropeptides by peripheral stimulation of identified frequencies. Physiology, 7(4), 176-180., Qureshi, I. S., Datta-Chaudhuri, T., Tracey, K. J., Pavlov, V. A., & Chen, A. C. (2020). Auricular neural stimulation as a new non-invasive treatment for opioid detoxification. Bioelectronic medicine, 6(1), 1-8., Jenkins, D. D., Khodaparast, N., O’Leary, G. H., Washburn, S. N., Covalin, A., & Badran, B. W. (2021). Transcutaneous Auricular Neurostimulation (tAN): A Novel Adjuvant Treatment in Neonatal Opioid Withdrawal Syndrome. Frontiers in Human Neuroscience, 15, 110.
Backed By A Level 1 Clinical Trial
Helping Support The Recovery Journey
Clinical trial participants also experienced reduction in Depression and PTSD
Copyright © 2024 Safe Science Solutions | All Rights Reserved